Health Professionals
Background Risk of Seizures, Strokes, and Myocardial Infarction
Compared to the Incidence of Such Events in Persons Consuming
Dietary Supplements Containing Ephedrine Alkaloids
Issue: Whether the incidence of seizures,
strokes, and myocardial infarctions (MIs) (i.e., heart attacks)
in users of dietary supplements containing ephedrine alkaloids is
higher than the background risk of such events in the general population.
Findings: FDA has failed to incorporate experience in the
general population to make an assessment of background risk into
its evaluation of ephedra safety and has therefore never determined
whether the reports it has received are anything more than events
that would have occurred even if ephedra had not been consumed.
Review of the available published literature shows that seizures
are a relatively common occurrence in young people, and that strokes
and MIs, while not "common," are not rare -- approximately 75,000
persons under the age of 45 have strokes per year in the U.S., and
approximately 22,000 to 66,000 persons under the age of 40 have
heart attacks.
Expert epidemiological analysis of available data shows that the
incidence of seizures, strokes, and MIs in persons consuming dietary
supplements containing ephedrine alkaloids is no greater than the
expected incidence in the general U.S. population. Scientific
assessment of background risks confirms that dietary supplements
containing ephedrine alkaloids are safe when consumed according
to current national industry standards.
Methodology: Dr. Stephen Kimmel, chair of the Ephedra Education
Council (EEC) Expert Panel, compared the incidence of seizures,
strokes, and MIs (hereinafter "events") in users of dietary supplements
containing ephedrine alkaloids to the incidence of events in the
general population. Dr. Kimmel estimated the number of events in
the general population by using incidence data obtained from recent
studies (i.e., articles published from 1985 to present). Dr. Kimmel
excluded certain studies from consideration in order to obtain the
most accurate rate of events.
Dr. Kimmel estimated the number of events among ephedra users by
using the number of events reported to the Food and Drug Administration's
(FDA's) Center for Food Safety and Applied Nutrition (CFSAN) from
June 1, 1997 to March 31, 1999. All reported events that were purportedly
related to exposure to ephedrine-containing dietary supplements
were included in the comparison even though there is no scientific
proof that the events were caused by ephedra ingestion. Further,
Dr. Kimmel included even those reports that FDA had conceded had
insufficient data from which to analyze the event and in which the
user abused or misused the product. Using all events in the estimate
(and not eliminating from consideration those with insufficient
data) provided an "upper-limit" of reported adverse events.
The true number of events among persons using ephedrine-containing
products is not known. To compensate for any possibility of underreporting,
Dr. Kimmel used a range of 1% - 20% of reported events in his analysis.
Similarly, the actual number of persons exposed to ephedrine-containing
dietary supplements is not known. Based on a survey of distributors
of these products, however, Dr. Kimmel used a conservative estimate
that approximately 2.8 to 11 million persons consume these products.
Numerous assumptions were made in determining the estimates of event
incidences and the number of product users. Every attempt was made
to overestimate the risk of events in ephedrine users.
Analysis
Seizures: Rate among Ephedra Users No Greater Than Rate among
General Population
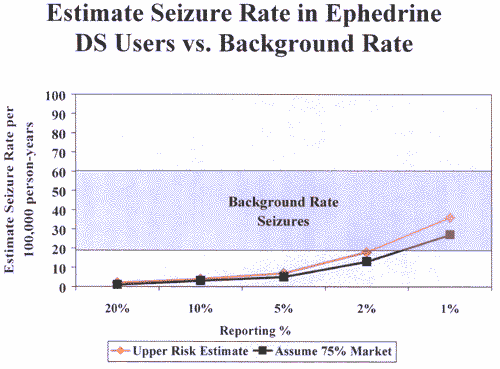
FDA received seven reports of seizures, and the age range of those
who experienced the seizures is 21 to 51. The background rate of
seizures in the population in this age range is 20 to 60 per
100,000. Using these estimates, Figure 1 (attached) demonstrates
that the estimated risk of seizures among ephedra users is within
the estimated range for background risk in the population. This
analysis shows no increased risk of seizures from consuming ephedra.
Stroke: Rate among Ephedra Users No Greater Than Rate among General
Population
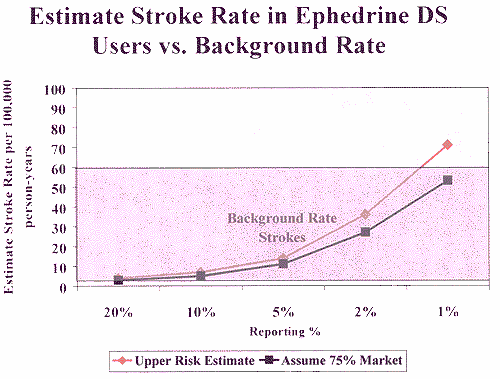
FDA received 14 reports of stroke, and the age range of those who
experienced a stroke is 18 to 64. Based on several U.S. studies,
the background rate of stroke in the population in this age range
is 7 to 60 per 100,000. Using these estimates, the incidence
of strokes among ephedra users is within the estimated range for
background risk in the population. (See Figure 2, attached.) This
analysis demonstrates that there is no increased risk of stroke
from consuming ephedra.
MIs: Rate among Ephedra Users No Greater Than Rate among General
Population
FDA received 11 reports of MIs, and the age range of those who experienced
an MI is 15 to 59. Based on two U.S. studies, the background rate
of MI in the population in approximately this age range is 5
to 41 per 100,000. Using these estimates, the incidence of MI
among ephedra users is within the estimated range for background
risk in the population. (See Figure 3, attached.) This analysis
indicates that there is no increased risk of MIs among ephedra users.
It is illogical for FDA to allege that dietary supplements containing
ephedrine alkaloids are causing seizures, strokes, and MIs when
the rates of those events among ephedra users are within the background
rates for those events in the general population. Only if the rates
of those events among ephedra users were higher than the background
rates for those events would FDA have cause for concern. Furthermore,
that young persons have experienced some of these events is tragic
but not surprising: approximately 75,000 persons under the age of
45 have strokes per year in the U.S., and approximately 22,000 to
66,000 persons under the age of 40 have heart attacks.
Conclusion: The use of dietary supplements containing ephedrine
alkaloids does not increase the risk of seizures, strokes, or heart
attacks.
In a February 10, 2000 memorandum, FDA's Center for Drug Evaluation
and Research (CDER) stated that "it is possible that the reported
serious adverse events are reflective of coincidental background
spontaneous occurrences in the population and are not necessarily
causally related to [the use of dietary supplements containing ephedrine
alkaloids.]" The above analysis, although in and of itself does
not conclusively prove that any particular event is or is not casually
related to ephedra, provides strong support for the conclusion that
use of dietary supplements containing ephedrine alkaloids does not
increase the risk of seizures, strokes or heart attacks.
Back to top
|



